Published On Premiered Jun 9, 2021
General Chemistry
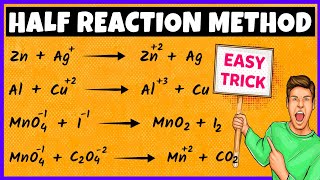
Balancing Redox Reaction Using Oxidation-Number-Change Method
One way to balance redox reactions is by keeping track electron transfer, by using the oxidation numbers of each of the atoms. For the oxidation-number-change method, start with the unbalanced skeleton equation.
Step 1: Assign oxidation numbers to each of the atoms in the equation and write the numbers above the atom.
Step 2: Identify the atoms that are oxidized and those that are reduced.
Step 3: Use a line to connect the atoms that are undergoing a change in oxidation number.
Step 4: Use coefficients to make the total increase in oxidation number equal to the total decrease in oxidation number.
Step 5: Check the balancing for both atoms and charge.
Learn Chemistry with Ma'am Cess
General Chemistry Tutorial Videos
https://www.youtube.com/watch?v=MOmk2...
For more updates, you can also follow my Facebook Page:
/ mathprofd
Join this channel to get access to perks:
https://www.youtube.com/channel/UCfOS...
For Business and Collaboration:
[email protected]
Please don't forget to like, share, and subscribe!
/ profd
Thank You Guys!
#LearnChemistryWithMaamCess #ProfD