Published On Sep 3, 2015
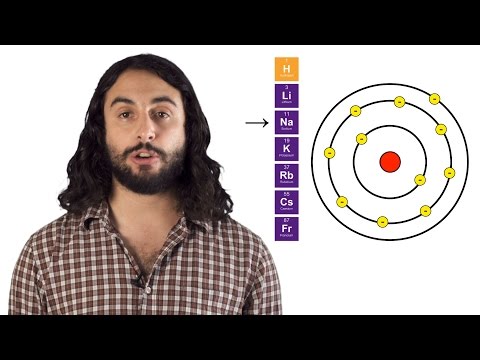
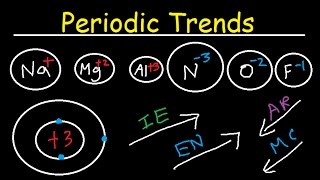
Why is the periodic table arranged the way it is? There are specific reasons, you know. Because of the way we organize the elements, there are special patterns that emerge. And you know how Professor Dave feels about patterns. He likes them.
Watch the whole General Chemistry playlist: http://bit.ly/ProfDaveGenChem
More AP Chemistry review materials from me: bit.ly/URPDave
Organic Chemistry Tutorials: http://bit.ly/ProfDaveOrgChem
Biochemistry Tutorials: http://bit.ly/ProfDaveBiochem
Biology Tutorials: http://bit.ly/ProfDaveBio
Classical Physics Tutorials: http://bit.ly/ProfDavePhysics1
Modern Physics Tutorials: http://bit.ly/ProfDavePhysics2
Mathematics Tutorials: http://bit.ly/ProfDaveMaths
EMAIL► [email protected]
PATREON► / professordaveexplains
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Amazon: https://amzn.to/2HtNpVH
Bookshop: https://bit.ly/39cKADM
Barnes and Noble: https://bit.ly/3pUjmrn
Book Depository: http://bit.ly/3aOVDlT